New skin on-chip model: microbiota integration into a skin microfluidic model and study of its effect. LMP233_21. Gobierno de Aragón. Proyectos de I+D+i en líneas prioritarias y de caracter multidisciplinar. (2021-2023)


P.I.: Jesús Ciriza Astrain
The regulation on drug development imposes an extremely long process (10 years on average) with enormous associated costs (2.6 billion dollars) and a success rate of 12%. Despite advances in basic research, translation of new targets into therapeutic approaches remains difficult and long-term. Consequently, the pharmaceutical industry is looking for reliable ways to improve productivity through new reliable decision-making strategies before starting expensive clinical trials. With the intention of bridging this wide gap between preclinical research and its translation into a marketable medical product, organ-on-chip (OoC) systems emerge as a promising approach to complement current preclinical approaches. OoC are capable of mimicking tissue human in vitro providing very reliable data on the response to drugs and/or possible medical interventions. One of the first tissue-engineered products to reach the market were the skin grafts, now widely used for the medical care of burn victims. These skin tissue models not only provide new insights into the mechanisms of skin development but are also widely used to test new skin care compounds. However, current models have only focused on simulating the physical barrier of the skin, a single part of the complex function of the skin. The epidermis of the skin is a tissue with a high regenerative potential due to the rapid renewal of cells promoted by epidermal stem cells. These cells continually produce new keratinocytes that undergo terminal differentiation to a keratinized layer and ultimately form the stratum corneum, the outer layer of the epidermis, made up of dead keratinocytes. Keratinocytes are embedded in a matrix of specialized lipids; their intracellular keratin filaments being tightly bound. To advance the potential of current skin models, a new generation of OoC is needed, replicating the complexities of human skin in a modern microfluidic system.
In this project, we propose to advance current in vitro cutaneous models towards a truly biomimetic model that includes the essential functions of this organ and its specific pathophysiological processes by including the microbiome on keratinocytes cultured on fibroblasts embedded in a matrix made up of human dermal extracellular matrix within a microfluidic system that simulates the dermal vasculature. The general objective of the project is to exploit an existing microfluidic system and advance to the next generation of complex in vitro skin models, incorporating the microbiome and the human extracellular matrix. The resulting technology is expected to lead to developments in skin-related products, both in the pharmaceutical and cosmetic sectors, as well as in clinical applications.
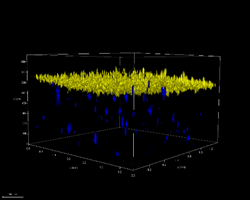

Microfluidic device with keratinocytes (yellow) on top of fibroblast (blue) embedded within a collagen hydrogel.

